Table Of Content
For example, in the above mentioned Melanoma and Tanning study, the researchers defined their population as any histologic variety of invasive cutaneous melanoma. However, they added another important criterion – these individuals should have a driver's license or State identity card. This probably is not directly related to the clinic condition, so why did they add this criterion? You would then collect data on your participants’ exposure to contaminated drinking water, focusing on variables such as the source of said water and the duration of exposure, for both groups. You could then compare the two to determine if there is a relationship between drinking water contamination and the risk of developing a gastrointestinal illness.
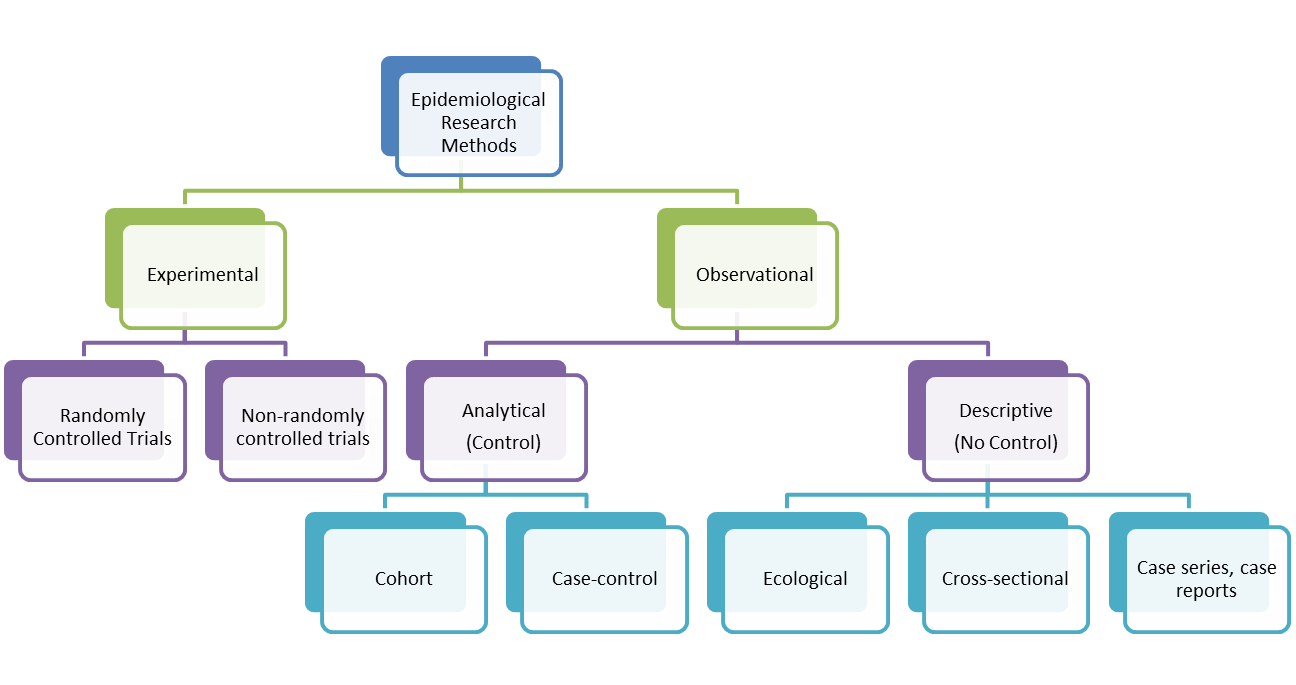
Research Design: Case-Control Studies
Case-control (case-control, case-controlled) studies are beginning to appear more frequently in the neurosurgical literature. They can be more robust, if well designed, than the typical case series or even cohort study to determine or refine treatment algorithms. The purpose of this review is to define and explore the differences between case-control studies and other so-called nonexperimental, quasiexperimental, or observational studies in determining preferred treatments for neurosurgical patients. In a retrospective cohort study, researchers examine a group before any of the subjects have developed the disease, then examine any factors that differed between the individuals who developed the condition and those who did not. The researcher would find a group of individuals with Kaposi's sarcoma (the cases) and compare them to a group of patients who are similar to the cases in most ways but do not have Kaposi's sarcoma (controls). The researcher could then ask about various exposures to see if any exposure is more common in those with Kaposi's sarcoma (the cases) than those without Kaposi's sarcoma (the controls).
Case-Control Study
The researcher would find a group of individuals with Kaposi's sarcoma (the cases) and compare them to a group of patients who are similar to the cases in most ways but do not have Kaposi's sarcoma (controls). The researcher might find that those with Kaposi's sarcoma are more likely to have HIV, and thus conclude that HIV may be a risk factor for the development of Kaposi's sarcoma. However, they can be a very efficient way of identifying an association between an exposure and an outcome.
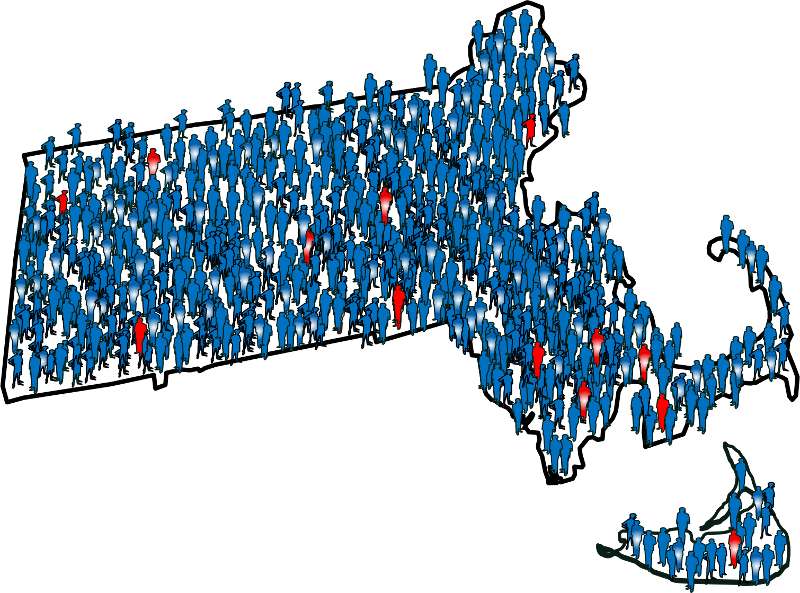
What Is a Case-Control Study? Definition & Examples
Similarly, if all of the cases of Kaposi's sarcoma were found to come from a small community outside a battery factory with high levels of lead in the environment, then controls from across the country with minimal lead exposure would not provide an appropriate control group. The investigator must put a great deal of effort into creating a proper control group to bolster the strength of the case-control study as well as enhance their ability to find true and valid potential correlations between exposures and disease states. If, for example, our cases of Kaposi's sarcoma came from across the country but our controls were only chosen from a small community in northern latitudes where people rarely go outside or get sunburns, asking about sunburn may not be a valid exposure to investigate. Similarly, if all of the cases of Kaposi's sarcoma were found to come from a small community outside a battery factory with high levels of lead in the environment, then controls from across the country with minimal lead exposure would not provide an appropriate control group.
Care must be taken to be objective in the search for past risk factors, especially since the outcome is already known, or the study may suffer from researcher bias. Although it may not always be possible, it is important to try to mask the outcome from the person who is collecting risk factor information or interviewing patients. Sometimes it will be necessary to interview patients about potential factors (such as history of smoking, diet, use of traditional eye medicines, etc.) in their past. Furthermore, patients who have the outcome (cases) are likely to scrutinize the past, remembering details of negative exposures more clearly than controls. Anything the researcher can do to minimize this type of bias will strengthen the study. Cohort and case–control studies aim to quantitatively estimate the association between a drug exposure and outcome.
This view emphasises that the threats to the validity of cohort studies should also be considered in case-control studies. For example, in case-control applications with risk-set sampling, researchers often consider the covariate and exposure status only at, or just before, the time of the event (for cases) or the time of sampling (for controls). However, where a cohort study would require information on baseline levels or the complete treatment and covariate history of participants, one should suspect that this holds for the nested case-control study too.
Goodness-of-fit two-phase sampling designs for time-to-event outcomes: a simulation study based on New York ... - BMC Medical Research Methodology
Goodness-of-fit two-phase sampling designs for time-to-event outcomes: a simulation study based on New York ....
Posted: Fri, 19 May 2023 07:00:00 GMT [source]
This type of design is useful for rare outcomes and those with long latent periods. However, they may also be prone to certain biases – selection bias and recall bias. This paper gives a formal account of how and when causal effects can be identified in case-control studies and, as such, underpins the case-control application of Dickerman et al. [3]. Like Dickerman et al., we believe that case-control studies should generally be regarded as being nested within cohort studies.
We emphasize that identification and estimation are distinct steps in causal inference. Although our focus was on the former, identifying functionals often naturally translate into estimators. The task of finding the estimator with the most appealing statistical properties is not necessarily straightforward, however, and is beyond the scope of this paper. Case-control studies compare groups retrospectively, while longitudinal studies can compare groups either retrospectively or prospectively.
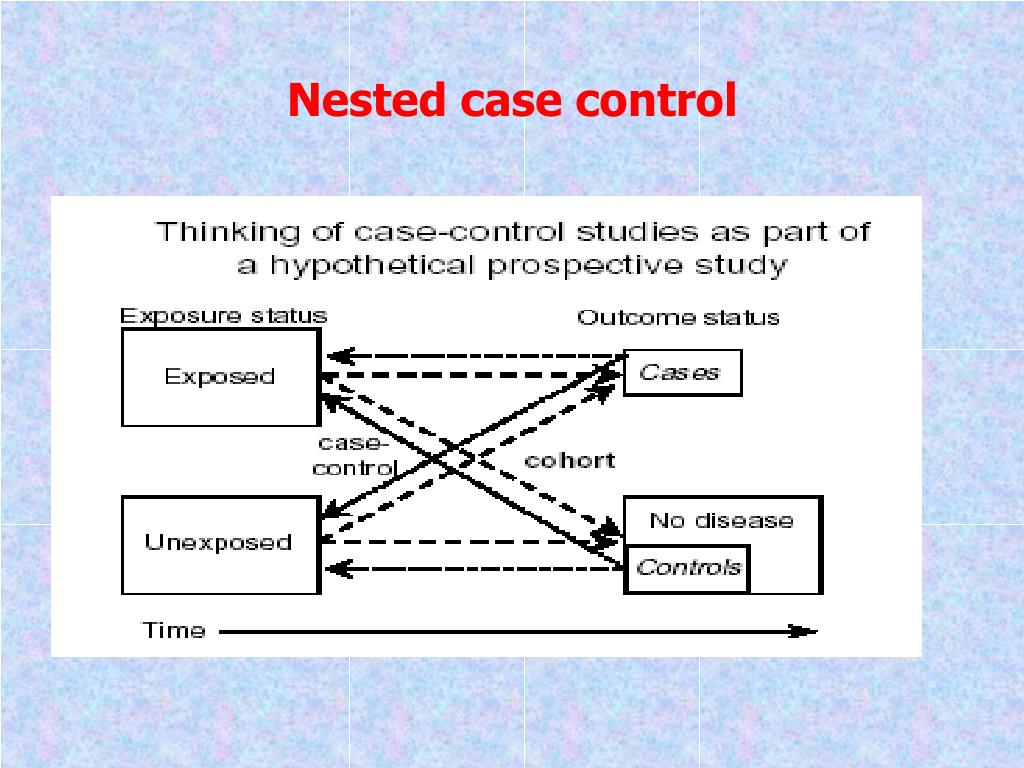
Case-control study nested in cohort study
COVID-19 associated bacterial infections in intensive care unit: a case control study Scientific Reports - Nature.com
COVID-19 associated bacterial infections in intensive care unit: a case control study Scientific Reports.
Posted: Wed, 16 Aug 2023 07:00:00 GMT [source]
Another use for case-control studies is investigating risk factors for a rare disease, such as uveal melanoma. Patients who present to hospital, however, may not be representative of the population who get melanoma. If, for example, women present less commonly at hospital, bias might occur in the selection of cases. (For identification of a conditional risk ratio, see Theorem 2 of Supplementary Appendix B.) Case-base sampling, also known as case-cohort sampling, means that no individual who is at risk at baseline of sustaining the event of interest is precluded from selection as a control.
Case-control studies, due to their typically retrospective nature, can be used to establish a correlation between exposures and outcomes, but cannot establish causation. These studies simply attempt to find correlations between past events and the current state. Case-control studies, due to their typically retrospective nature, can be used to establish a correlation between exposures and outcomes, but cannot establish causation.
As seen in Figure 1, at the time of entry into the study (sampling of participants), some of the study participants have the outcome (cases) and others do not have the outcome (controls). During the study procedures, we will examine the exposure of interest in cases as well as controls. We will then study the association between the exposure and outcome in these study participants. Bias in a case-control study might also result if exposures cannot be measured or recalled equally in both cases and controls. Healthy controls, for example, may not have been seen by a physician for a particular illness or may not remember the details of their illness. Choosing from a population with a disease different from the one of interest but of similar impact or incidence may minimize recall and measurement bias, since affected individuals may be more likely to recall exposures or to have had their information recorded to a level comparable to cases.
The authors have done a nice job of explaining the fundamental concepts of an underutilized but very useful retrospective study design, the case control study. As they point out, because many neurosurgical conditions are relatively uncommon, and because we are impatient to answer important clinical questions, the sample sizes and duration of prospective cohort studies and randomized trials may be impractical for many neurosurgical questions. Case-control study, in epidemiology, observational (nonexperimental) study design used to ascertain information on differences in suspected exposures and outcomes between individuals with a disease of interest (cases) and comparable individuals who do not have the disease (controls). Analysis yields an odds ratio (OR) that reflects the relative probabilities of exposure in the two populations.
Sample size calculations required for statistical significance typically yield far fewer subjects than necessary for enrollment in RCTs,5 which is beneficial in studying uncommon conditions and outcomes. This is because in case-control studies, groups are divided by the outcome presence or absence looking backward for prevalence of exposure, treatment, or risk factors. In cohort studies, on the other hand, groups are divided by exposure, treatment or risk factors, looking forward to frequency of outcomes. Because frequency of outcomes is almost always smaller than prevalence of exposure, treatment, or risk factors, sample sizes in cohort studies are (usually) much larger than those of case-control studies.
At $495, the DaVinci Resolve Micro Color Panel is significantly more affordable than its larger counterparts. This opens the door for a wider range of editors and colorists to experience the power of dedicated control panels and take their creative output to the next level. For existing DaVinci Resolve users, the Micro Color Panel offers a familiar feel. The trackballs boast a similar professional design, and the shift keys mimic the layout of higher-end panels.


No comments:
Post a Comment